Silicones help make products more sustainable in several markets, including the transportation, electronics, energy, and consumer product sectors.
Silicones Environmental, Health, and Safety Center (SEHSC)

The Silicones Environmental, Health, and Safety Center is a sector group of the American Chemistry Council (ACC). SEHSC promotes the safe use of silicones through product stewardship, outreach, and environmental, health, and safety research.
SEHSC is managed in the Chemical Products and Technology Division (CPTD) of the American Chemistry Council, which provides technical and management services, issue management activities, specialized advocacy, research, education, communication, and evaluation services to a core group of more than 50 sector and product groups.
SEHSC works with allied organizations in Europe Silicones Europe and Japan (SIAJ). Efforts of these associations are coordinated through the Global Silicones Council.
Uses & Benefits
- Solar Panels
- Wind Turbines
- Sealants
- Adhesives
- Coatings
- Electrical Devices & Wiring
- Airbags
- Fuel Efficiency
- Keypads & Keyboards
- Cell phones
- Medical Tubing
- Lubricants
- Prosthetics
- Ventilation masks
- Respirators
Key Ways Silicones is Transforming Your Life
Revolutionizing Construction
Silicone products used in construction help protect, strengthen, preserve, and provide aesthetic/innovative features to buildings.
Learn moreProviding Energy Savings
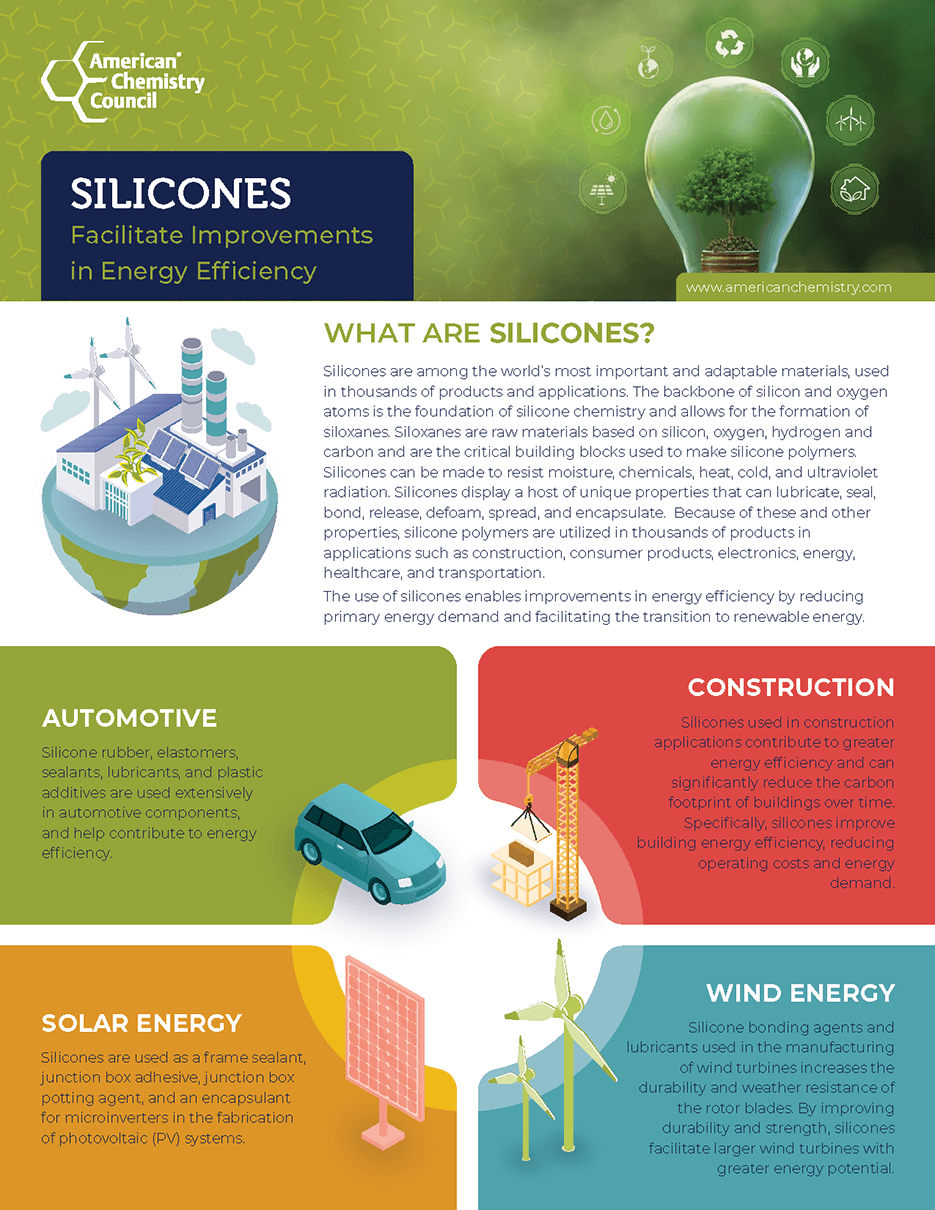
Silicones provide significant energy savings and are used in wind turbines and solar panels to power the transition to renewable energy.
Learn moreIncreasing Fuel Efficiency
Silicone components contribute to weight reduction in automobiles, resulting in increased fuel efficiency.
Learn moreEnabling Healthcare Innovation
Silicones seal, bond, cushion, insulate, and facilitates many innovative medical technologies.
Learn moreSilicone Chemistry Infographics
- Silicone Agriculture Applications
- Silicones Facilitate Improvements in Energy Efficiency
- Silicones Facilitate a Transition to a Carbon-Neutral Economy
- Silicones Enabling Renewable Energy and Energy Efficiency
- Silicone Medical Applications
- Silicone Transportation Equipment Application
- Silicone Automotive Applications
- Silicone Textile Applications
- Silicone Electrical Machinery, Electronics and Telecommunication Applications
- Silicone Building and Construction Applications